There are two kinds of reactions. First is known as chemical reaction and second is known as physical reaction. The process of writing the chemical equation in the form of symbols and formulae is known as a chemical equation. There are two sides of a chemical equation. On the left side of a chemical equation, we write reactants and on the right side of a chemical equation, we write products. In order to separate the reactants and products, we use an arrowhead. The tail of the arrow is towards reactants and the head of the arrow is towards products. While writing a chemical equation, we should ensure that the number of atoms on the reactant side should equal the number of atoms on the product side. An example of a chemical equation is given below; Image The above example shows that when Hydrogen and Oxygen mix up, they form water.
Types of Chemical Equations
There are two types of chemical equations. The first of known as the balanced chemical equation and the second is known as the unbalanced chemical equation.
1. Balanced Chemical Equation
A chemical equation that has the same number of atoms on both sides of the equation is known as a balanced chemical equation. In other words, in a balanced chemical equation, the numbers of atoms on the reactant side are equal to the number of atoms on the product side. In the following image, there is an example of a balanced chemical equation.
The above equation shows that we have one Carbon atom, four Hydrogen atoms, and four Oxygen atoms on the reactant side. In a similar way, we have also the same number of atoms on the product side. Therefore, it is an example of a balanced chemical equation.
2. Unbalanced Chemical Equation
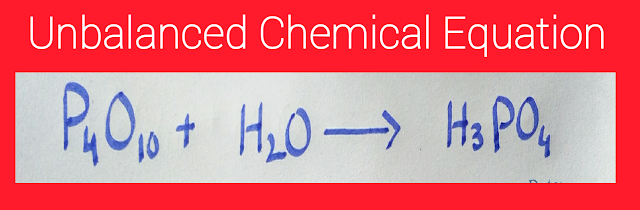
A chemical equation in which the numbers of atoms on both sides of the equation are not the same is known as an unbalanced chemical equation. In other words, we can say that the numbers of atoms on the reactant side are not equal to the number of atoms on the product side. In the following image, there is an example of an unbalanced chemical equation;
The above equation is an unbalanced chemical equation. Its reason is that on the reactant side, there are four P atoms, eleven O atoms, and two H atoms. On the other hand, on the product side, there are three H atoms, one P atom, and four O atoms. As the numbers of atoms on the reactant side are not equal to the number of atoms on the product side, therefore, it is an unbalanced chemical equation.
You can also read: How does ice float on water?
How to Balance Chemical Equations
While studying, we have to come across lots of unbalanced chemical equations. Therefore, it is necessary for us to balance these chemical equations. In order to balance these chemical equations, you will have to follow these essential steps;
♦ In the first step, you must write the given equation in its original form.
♦ In the second step, you will have to note the number of atoms. For this reason, you should note the number of atoms on each side of the equation. It means that you should write the number of atoms on the reactant side on the left side of the paper and the number of atoms on the product side on the right side of the equation.
♦ While balancing the equation, you should get an idea of whether there are O and H atoms in the equation or not. If there are H and O atoms in the equation, you should try to balance these atoms at last.
Further Steps
♦ If you have only one element to balance on each side of the equation, it is good because you can easily balance it. On the other hand, if you have to balance more than one atom on each side of the equation, you should treat these atoms simultaneously. It means that first of all, you should take one atom and balance it on both sides of the equation. After balancing the first atom, you should move toward another atom. In a similar way, you should try to balance all the atoms in your equation.
♦ While balancing atoms on each side of the equation, you can use coefficients. For example, if you have to put 3 as the coefficient on one side of the equation and the total number of atoms becomes 6, you should also try to adjust such coefficient on the other side of the equation which makes the 6 same atoms.
♦ After balancing all the other atoms, you should try to balance the H atoms by following the same rule.
♦ At the end, you will have to balance the O atoms.
If you follow these simple steps, it will be easy for you to balance any kind of chemical equation.
How to Balance Chemical Equations With Example
After clearly understanding the rules to balance a chemical equation, we are in a position to balance any kind of unbalanced chemical equation. Here, we will discuss how to balance a chemical equation by following these rules with the help of an example.
♦ In the first step, we write the given equation in its original form in the following way.
♦ In the second step, we note down all the numbers of atoms on each side of the chemical equation in the following way;
| Reactant | Product |
| P | 4 | 1 |
| H | 2 | 3 |
| O | 11 | 4 |
♦ As there are O and H atoms in this unbalanced equation. Therefore, we will ignore these atoms and try to balance these atoms at last.
♦ First of all, we take the P atom and try to balance it on both sides of the equation. As there are four P atoms on the reactant side, therefore, if we want to balance it on the product side, we will have to put coefficient four in the following way;
♦ Now, we have to balance H atoms. After putting coefficient 4 on the product side, there are two H atoms on the reactant side and twelve H atoms on the product side. Therefore, we have to put coefficient 6 on the reactant side in the following way;
♦ At the end, we have to balance O atoms. After putting these coefficients, we can see that there are 16 Oxygen atoms on both sides of the equation. It means that we don’t need to do anything to balance O atoms on both sides of the equation.
♦ Now, the required balanced equation is given below;
| Reactant | Product |
| P | 4 | 4 |
| H | 12 | 12 |
| O | 16 | 16 |












2 thoughts on “How to Balance Chemical Equations”